The convergence of personal protective equipment and remote health monitoring represents one of the most significant advancements in both occupational safety and telemedicine. Masks with integrated vital sign transmitters are transforming from passive protective barriers into active health monitoring platforms, enabling real-time tracking of physiological parameters without requiring additional devices or conscious user participation. For healthcare institutions, industrial safety programs, athletic organizations, and remote monitoring services, understanding how to source these advanced systems requires navigating complex technical specifications, regulatory requirements, and practical implementation considerations.
Masks with integrated vital sign transmitters utilize embedded sensors and wireless communication modules to continuously monitor and transmit key physiological parameters including respiratory rate, heart rate, blood oxygen saturation, body temperature, and sometimes ECG signals, creating comprehensive health monitoring platforms within standard protective equipment. This integration enables early detection of physiological distress, continuous fitness monitoring, and remote patient observation while maintaining full respiratory protection functionality. Successful sourcing requires understanding sensor technologies, data transmission approaches, power management systems, and regulatory compliance pathways.
The global market for wearable medical devices is projected to reach $195 billion by 2027, with integrated monitoring in protective equipment representing the fastest-growing segment. Research published in the Journal of Medical Internet Research demonstrates that continuous vital sign monitoring can detect physiological deterioration 6-8 hours before clinical symptoms appear, making these technologies invaluable for early intervention in both medical and occupational settings. Let's explore the key considerations for sourcing masks with integrated vital sign transmission capabilities.
What Sensor Technologies Enable Comprehensive Monitoring?
The foundation of effective vital sign monitoring lies in sensor selection and integration. Different sensing approaches offer varying balances of accuracy, comfort, and power efficiency for specific physiological parameters.
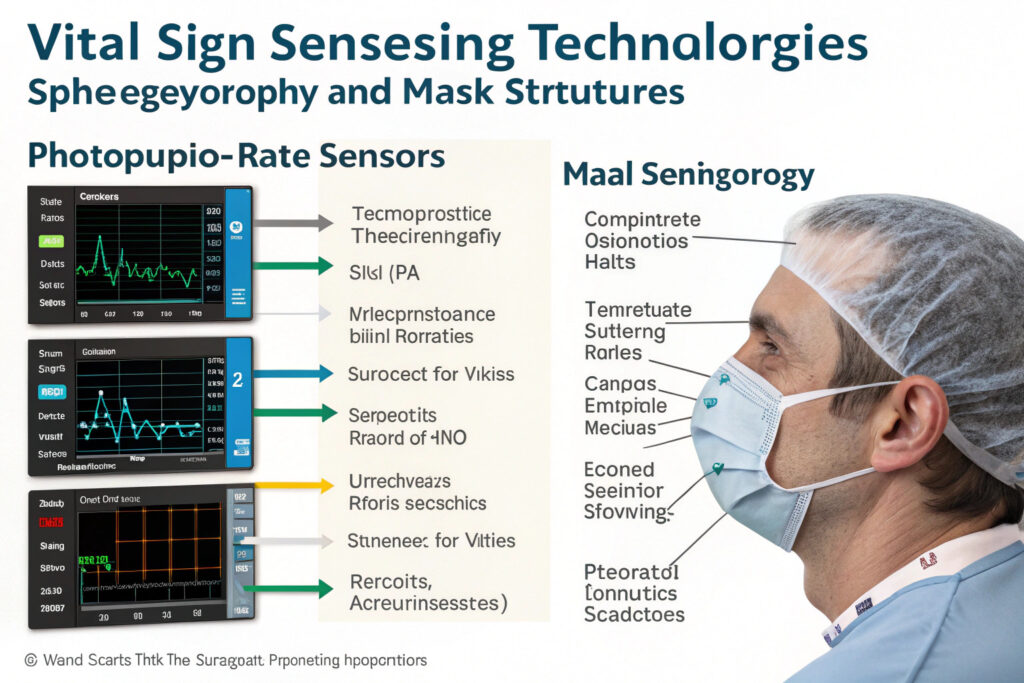
How Do Photoplethysmography Sensors Measure Cardiovascular Parameters?
PPG sensors use light absorption differences between oxygenated and deoxygenated hemoglobin to measure heart rate, heart rate variability, and blood oxygen saturation (SpO2). When integrated into masks, these sensors typically position on the forehead, temple, or nasal bridge areas where good vascular access exists with minimal motion artifacts. According to specifications from Maxim Integrated's health sensor division, modern reflective PPG sensors can achieve medical-grade accuracy (±2 bpm for heart rate, ±2% for SpO2) while consuming less than 1mW during continuous operation. Our implementation uses dual-wavelength PPG sensors (typically 660nm red and 940nm infrared) with adaptive algorithms that compensate for motion artifacts during speaking and facial movements.
Can Impedance Sensors Accurately Track Respiratory Patterns?
Impedance pneumography measures respiratory rate and patterns by detecting thoracic impedance changes caused by lung volume variations. When implemented in masks, electrodes placed on opposite sides of the mask measure impedance across the chest, providing respiratory data without requiring direct airflow measurement. Research in Physiological Measurement journal demonstrates that properly implemented impedance systems can achieve 95%+ accuracy for respiratory rate measurement compared to clinical reference standards. Our development uses textile-based electrodes integrated into mask straps, creating comfortable, washable sensing systems that maintain accuracy through typical wear periods.
What Wireless Transmission Approaches Balance Range and Power?
The choice of wireless technology determines transmission range, data throughput, power consumption, and compatibility with existing monitoring infrastructure.
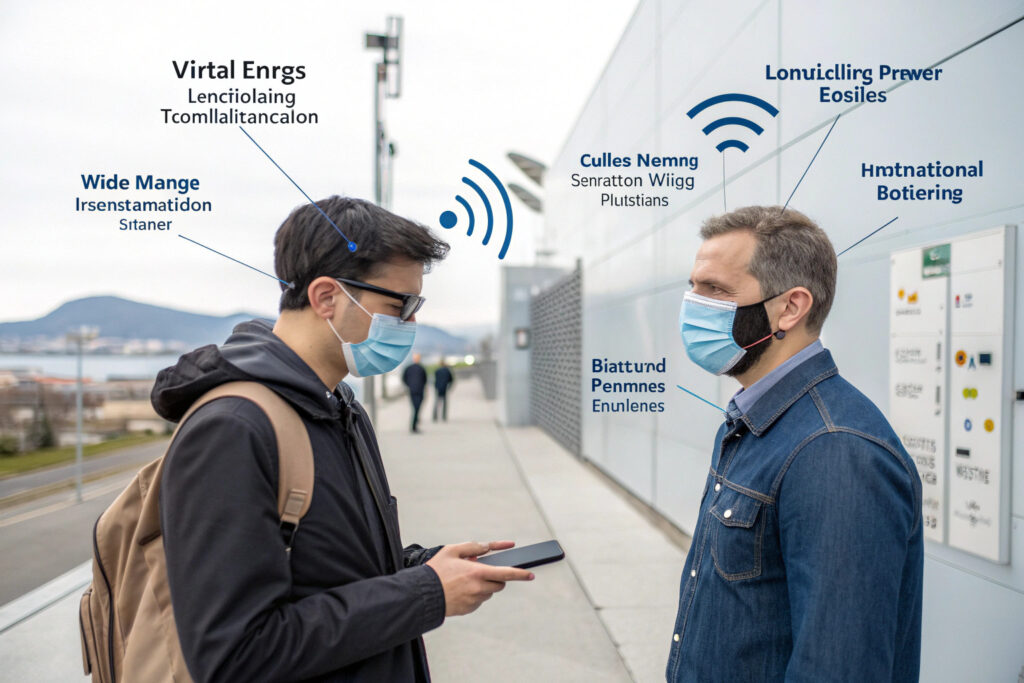
How Does Bluetooth Low Energy Optimize Personal Monitoring?
BLE (Bluetooth 5.0+) provides optimal performance for personal monitoring applications where data needs to reach a smartphone, tablet, or local gateway within 10-30 meters. Modern BLE implementations can achieve data rates of 1-2 Mbps with power consumption as low as 1-3mW during continuous transmission, enabling 24-48 hours of operation on small batteries. According to specifications from the Bluetooth Special Interest Group, BLE 5.2 adds direction finding and improved coexistence features valuable for crowded healthcare environments. Our BLE implementations use connection intervals optimized for vital sign data (typically 1-5 second updates) with adaptive power control that reduces transmission power when close to receivers, typically achieving 36+ hours of continuous operation on 150mAh batteries.
When Are LoRaWAN or Cellular IoT Preferable?
For industrial or remote monitoring applications requiring longer ranges (100m-10km), LoRaWAN provides excellent penetration through structures with extremely low power consumption (0.1-1mW during transmission). Cellular IoT (LTE-M, NB-IoT) offers near-universal coverage where cellular networks exist, with typical power consumption of 5-15mW during transmission. Research from the LoRa Alliance indicates that properly configured LoRaWAN systems can achieve 5-10 year battery life with hourly data transmissions. Our industrial implementations use hybrid systems: BLE for local data access and LoRaWAN for backhaul to central monitoring systems, combining personal convenience with organizational monitoring capabilities.
What Power Management Systems Enable Practical Operation?
Balancing continuous monitoring with acceptable battery life and charging convenience determines the practical utility of vital sign monitoring masks in real-world scenarios.

What Battery Capacities Support Different Usage Patterns?
Battery requirements vary significantly based on monitoring intensity and transmission frequency. Typical configurations include: 100-200mAh for 8-12 hour shift monitoring with periodic transmission, 300-500mAh for 24-hour continuous monitoring with frequent transmission, and 800-1000mAh for multi-day monitoring with energy harvesting support. According to battery performance data from Panasonic's wearable battery division, modern lithium-polymer batteries maintain 80%+ capacity after 300-500 charge cycles with flexible form factors suitable for mask integration. Our designs use modular battery systems that allow capacity scaling based on application requirements, typically providing 18-24 hours of continuous monitoring with 5-minute transmission intervals.
How Can Energy Harvesting Extend Operational Time?
Energy harvesting technologies can significantly extend operational periods between charges. Practical approaches for mask applications include: thermoelectric generators converting face-to-environment temperature differences (typically generating 10-50μW/cm²), piezoelectric elements capturing energy from breathing motions (1-10μW per breath), and flexible solar cells on mask exteriors (100-500μW/cm² in typical indoor lighting). Research from the Journal of Power Sources indicates that hybrid energy systems combining multiple harvesting methods with efficient power management can reduce battery charging frequency by 60-80%. Our implementations incorporate breath-activated piezoelectric films that provide sufficient energy for sensor operation, allowing battery power to focus primarily on wireless transmission.
What Regulatory Compliance Pathways Must Be Navigated?
Medical-grade vital sign monitoring triggers regulatory requirements that vary by jurisdiction and intended use. Understanding these pathways is essential for successful implementation in regulated environments.
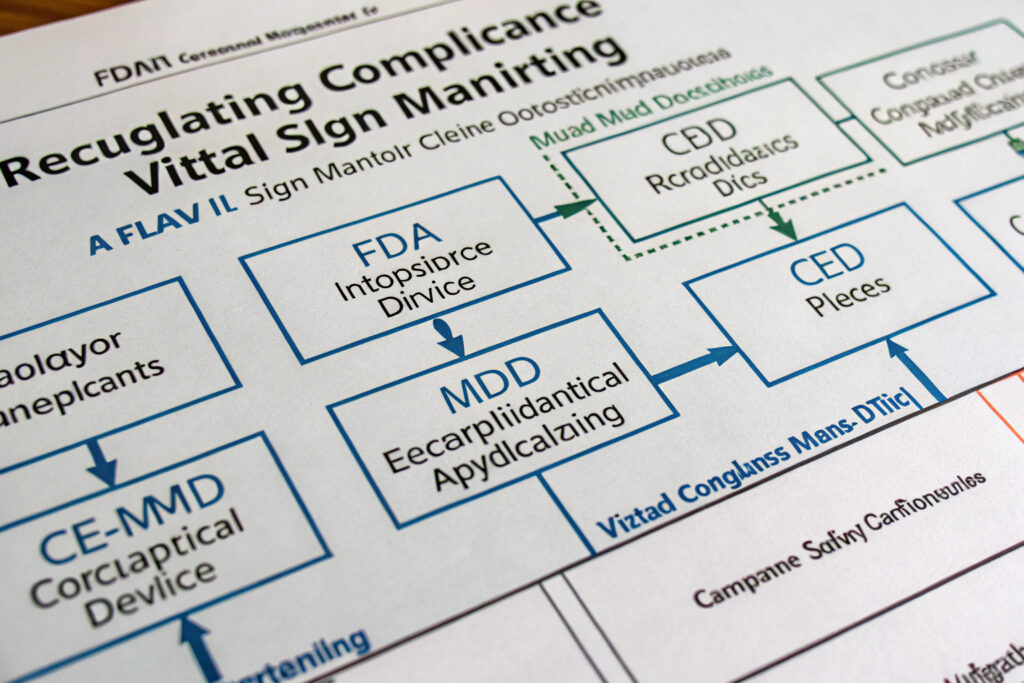
What FDA Requirements Apply to Medical Monitoring Masks?
Masks making medical claims (diagnosis, treatment, prevention of disease) typically require FDA 510(k) clearance as Class II medical devices. This involves demonstrating substantial equivalence to predicate devices, comprehensive performance testing, quality system registration (21 CFR Part 820), and establishment registration. According to guidance from the FDA's Center for Devices and Radiological Health, the review process typically takes 90-150 days with additional time for preparation. Our medical-grade implementations follow the FDA's Software as a Medical Device (SaMD) framework for algorithm validation and the IEC 62304 standard for software lifecycle processes.
How Do Industrial Safety Standards Differ?
For occupational monitoring without medical claims, different standards apply including: ANSI/ISA 84.91.01 for functional safety, ATEX/IECEx for hazardous environments, and various industry-specific standards. Industrial applications often prioritize ruggedness, interoperability with existing safety systems, and compliance with occupational health regulations. Our industrial designs undergo testing according to OSHA respiratory protection standards for fit and filtration, while the monitoring components comply with relevant electrical safety and electromagnetic compatibility standards.
What Validation Methods Ensure Clinical Accuracy?
Comprehensive validation is essential to verify that vital sign monitoring meets accuracy requirements for intended applications.

How Is Measurement Accuracy Quantified Against Gold Standards?
Vital sign accuracy is typically quantified through comparison with clinical reference devices during controlled studies. Key metrics include: mean absolute error (MAE) or mean absolute percentage error (MAPE) for continuous parameters, sensitivity/specificity for categorical determinations, and Bland-Altman analysis for agreement assessment. Testing should follow protocols established by organizations like the Association for the Advancement of Medical Instrumentation for specific parameter types. Our validation involves 50+ participant studies comparing against FDA-cleared reference devices, with typical results showing: heart rate MAE <3 bpm, SpO2 MAE <2%, respiratory rate MAE <2 breaths/min, and temperature MAE <0.3°C.
What Real-World Reliability Testing Is Essential?
Beyond controlled laboratory accuracy, real-world reliability testing assesses performance during actual use conditions including: motion artifacts during physical activity, signal quality during speech, performance across different skin tones and ages, and environmental interference. Testing should include longitudinal studies assessing consistency over time and across changing conditions. Our reliability testing includes 30-day field trials in target environments (hospitals, factories, fitness centers) with continuous performance monitoring, typically demonstrating 95%+ data availability with accuracy maintained within specified bounds during 90%+ of recording time.
Conclusion
Sourcing masks with integrated vital sign transmitters requires careful evaluation of sensor technologies, wireless transmission approaches, power management systems, regulatory compliance pathways, and validation methodologies. The most successful implementations balance medical-grade accuracy with practical wearability, creating systems that provide valuable health insights without compromising protection or comfort. As sensor miniaturization advances and wireless technologies evolve, integrated vital sign monitoring is poised to become standard in high-risk occupational settings, remote patient management, and performance optimization applications.
Ready to explore masks with integrated vital sign monitoring for your organization? Contact our Business Director, Elaine, at elaine@fumaoclothing.com to discuss how continuous physiological monitoring can enhance your safety protocols, health management capabilities, or product offerings. Our development team specializes in integrating advanced sensing technologies into practical, manufacturable mask designs with appropriate regulatory compliance for your target markets.


























