The integration of novel nanomaterials into consumer and industrial products represents a frontier of innovation, but it also introduces complex regulatory obligations. In the United States, the Toxic Substances Control Act (TSCA) serves as the primary federal law governing the introduction and use of new chemical substances, including engineered nanomaterials. For manufacturers developing next-generation products like advanced filtration membranes, smart coatings, or conductive textiles, understanding how to navigate TSCA's requirements is not just about compliance—it's a critical step in responsible commercialization and risk management.
Navigating TSCA requirements for novel nanomaterials involves a multi-step process of determining if your material is considered a "new chemical substance," preparing a Premanufacture Notice (PMN) or another applicable submission for the Environmental Protection Agency (EPA), and generating or providing robust health and environmental safety data to support a finding of "no unreasonable risk" as mandated under the 2016 Lautenberg Act amendments. The process is particularly nuanced for nanomaterials because their unique properties may trigger distinct considerations beyond their bulk chemical counterparts. Successful navigation requires early planning, scientific rigor, and often, strategic engagement with the EPA.
The EPA has significantly increased its focus on nanomaterial regulation under TSCA, with several existing chemical substances now having specific nanoscale reporting and recordkeeping rules under Section 8(a). For companies at the forefront of material science, proactive and thorough engagement with TSCA is essential to avoid significant delays, costs, and potential enforcement actions. Let's break down the practical pathway.
Is Your Nanomaterial Considered "New" or "Existing" Under TSCA?
The first and most critical question determines the entire regulatory pathway. The answer hinges on whether the nanomaterial is listed on the TSCA Inventory.

How Does the EPA Define a "New" Nanomaterial?
A chemical substance is "new" if it is not listed on the public TSCA Chemical Substance Inventory. For nanomaterials, the EPA generally considers:
- A distinct molecular identity: If the nanomaterial has a specific molecular structure that differs from its bulk form (e.g., a specific fullerenes like C60), it may be considered a unique substance.
- A distinct "discrete form": The EPA's 2023 Framework for Nanomaterials under TSCA indicates that certain nanoscale forms of existing chemicals may be considered "different substances" if they exhibit unique properties. This is a complex, case-by-case determination.
If your engineered nanomaterial (e.g., a specific graphene oxide with a defined oxygen content and sheet size, or a surface-functionalized quantum dot) is not explicitly listed, you should assume it is "new" and requires a PMN submission before manufacture or import for a non-exempt commercial purpose.
What if the "Bulk" Chemical is Listed, but the Nano-Form is Not?
This is a common gray area. The EPA has issued Significant New Use Rules (SNURs) for several existing chemicals (like carbon nanotubes, silver, and cerium oxide) when used in nanoscale form. A SNUR designates the nanoscale use as a "significant new use," requiring anyone wishing to manufacture or import the chemical for that use to submit a Significant New Use Notice (SNUN) at least 90 days before commencing activity. The SNUN process is similar to a PMN. Therefore, you must check both the Inventory and the relevant SNURs in 40 CFR Part 721.
What is the Premanufacture Notice (PMN) Process for Nanomaterials?
The PMN is the standard vehicle for introducing a new chemical substance. For nanomaterials, the submission must be particularly detailed to address potential unique risks.

What Specific Data and Information Must Be Included?
A complete PMN (Form 7710-25) requires extensive information. For nanomaterials, the EPA's guidance and its Information Requirements for Nanoscale Materials are crucial. Key sections include:
- Chemical Identity and Characterization: Precise details on morphology (shape, size, size distribution, aggregation state), surface chemistry (functionalization, charge, area), and crystal structure. Analytical methods (TEM, SEM, DLS, XRD data) are critical.
- Physical-Chemical Properties: Not just standard properties, but those relevant to nano: dispersibility, dustiness, photocatalytic activity, solubility/dissolution rate in relevant media.
- Health and Environmental Hazard Data: The EPA may require or you may need to proactively generate:
- Environmental Fate: Adsorption/desorption, degradation, bioaccumulation potential.
- Ecotoxicology: Data on aquatic and terrestrial organisms (e.g., daphnia, algae, fish).
- Toxicology: In vitro or in vivo data on pulmonary toxicity, dermal irritation, mutagenicity (Ames test). For materials likely to be inhaled (like mask filter media), pulmonary toxicity studies are often a key focus.
- Exposure and Use Information: Detailed description of the manufacturing process, industrial/commercial uses, expected worker and consumer exposure scenarios, and disposal methods.
The 2016 TSCA amendments require the EPA to make an affirmative finding that the new chemical does not present an unreasonable risk to health or the environment. Insufficient data will lead to a "risk-based" determination, often resulting in a restrictive Consent Order.
How Can the Exposure and Use Information Reduce Data Demands?
A well-defined, controlled use scenario is your strongest tool for a successful PMN. If you can demonstrate low exposure potential, you may justify a reduced data set. For a nanomaterial encapsulated within a solid polymer matrix in a mask filter (where release is extremely unlikely), the exposure argument is far stronger than for a nanomaterial used in a sprayable coating. Clearly articulating engineering controls (closed manufacturing systems) and product stewardship plans (safe handling instructions) in the PMN is essential.
What Are the Exemptions and Alternative Pathways?
Not all new nanomaterials require a full PMN. Several exemptions exist, but they come with strict criteria and reporting obligations.
Is the R&D Exemption Applicable?
The TSCA R&D exemption (40 CFR § 720.36) allows for the manufacture and import of a new chemical substance solely for research and development without a PMN, provided it is under the control of a qualified technician and all waste is disposed of properly. This is vital for pilot-scale production and testing. However, distribution for commercial testing (e.g., sending samples to potential customers) generally does NOT qualify. The line between R&D and commercial activity is strictly enforced.
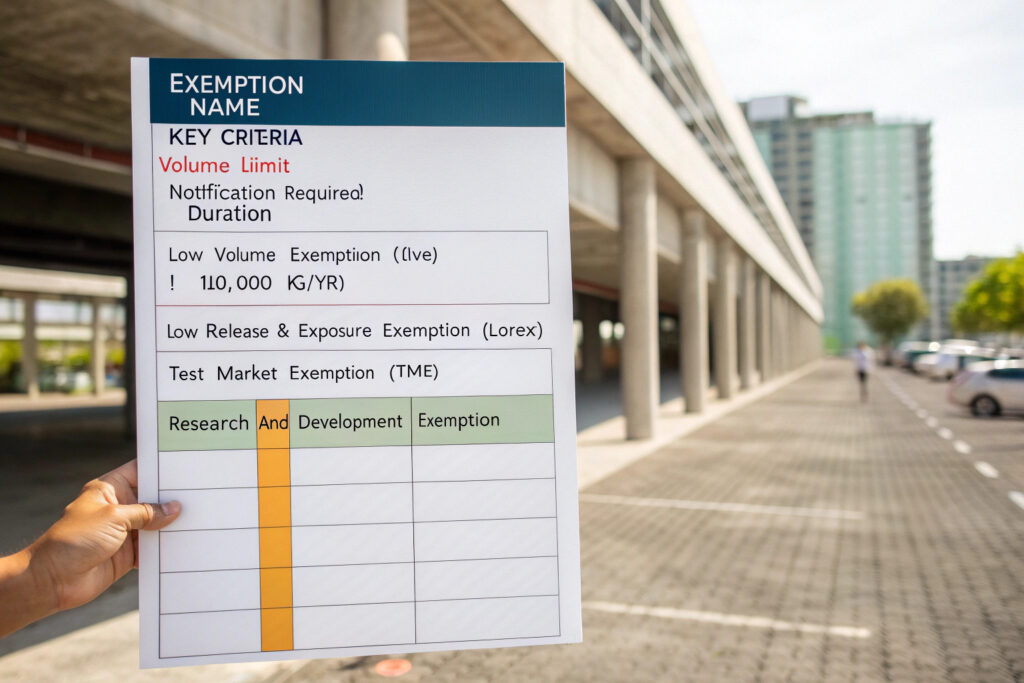
What are the Low Volume and Low Exposure Exemptions?
- Low Volume Exemption (LVE): For manufacture ≤ 10,000 kg/year. Requires a submission (Form 7710-125) and may still require some hazard data. A good option for scaling up a niche, high-value nanomaterial.
- Low Release and Low Exposure Exemption (LoREX): For substances where exposure is rigorously controlled. Difficult to achieve for powders or materials in dispersions but potentially feasible for fully encapsulated nanomaterials.
These exemptions require a submission (less extensive than a PMN) and EPA review, but they can be faster pathways for limited production.
What are the Post-Notification and Ongoing Obligations?
Successful PMN review does not end your TSCA obligations. The outcome dictates your long-term compliance duties.
What is a "Consent Order" and What Does it Require?
It is common for novel nanomaterials to receive a Consent Order under TSCA Section 5(e). This is a legally binding agreement that allows manufacture under specific conditions designed to mitigate risk. Conditions may include:
- Limits on Use: Restricting the substance to specific applications (e.g., "only for use bound in solid polymer matrices").
- Workplace Protection: Requiring specific personal protective equipment (PPE) and engineering controls for handlers.
- Environmental Release Controls: Dictating wastewater treatment or air emission controls.
- Hazard Communication: Specific labeling and Safety Data Sheet (SDS) requirements.
- Post-Manufacture Testing: Requiring the submitter to generate and submit additional health or environmental effects data within a specified timeframe.
You must comply with every condition in the Order. Violations can lead to significant penalties.
What are the Ongoing Reporting Rules (CDR, SNUR)?
- Chemical Data Reporting (CDR): If you manufacture or import the nanomaterial at volumes ≥ 25,000 lb/year (approx. 11,340 kg/year) at a single site, you must report production volume and use information every four years.
- Significant New Use Rules (SNUR): The EPA may later issue a SNUR for your substance if other companies want to use it in ways that were not assessed in your PMN. You must monitor the Federal Register for such rules.
- Adverse Effects Reporting: You have a duty to report any new information that reasonably supports the conclusion that the substance presents a substantial risk (TSCA Section 8(c)).
Conclusion
Navigating TSCA for novel nanomaterials is a rigorous, data-driven, and iterative process. It begins with a careful determination of the substance's status on the Inventory, proceeds through a detailed PMN preparation focusing on nanoscale characterization and exposure scenarios, and culminates in ongoing compliance with EPA orders and reporting rules. The key to efficiency is early engagement: consult the EPA's guidance documents, consider seeking a pre-submission conference with the EPA's New Chemicals Division, and build a robust safety data package tailored to the unique properties and intended uses of your nanomaterial. While the path is complex, successfully navigating it not only ensures legal market access but also builds a foundation of safety and responsibility that is critical for public trust and sustainable innovation.
Ready to develop a strategic plan for TSCA compliance for your novel nanomaterial application? Contact our Business Director, Elaine, at elaine@fumaoclothing.com to discuss how we can assist with regulatory strategy, data gap analysis, and preparation of submissions to the EPA.