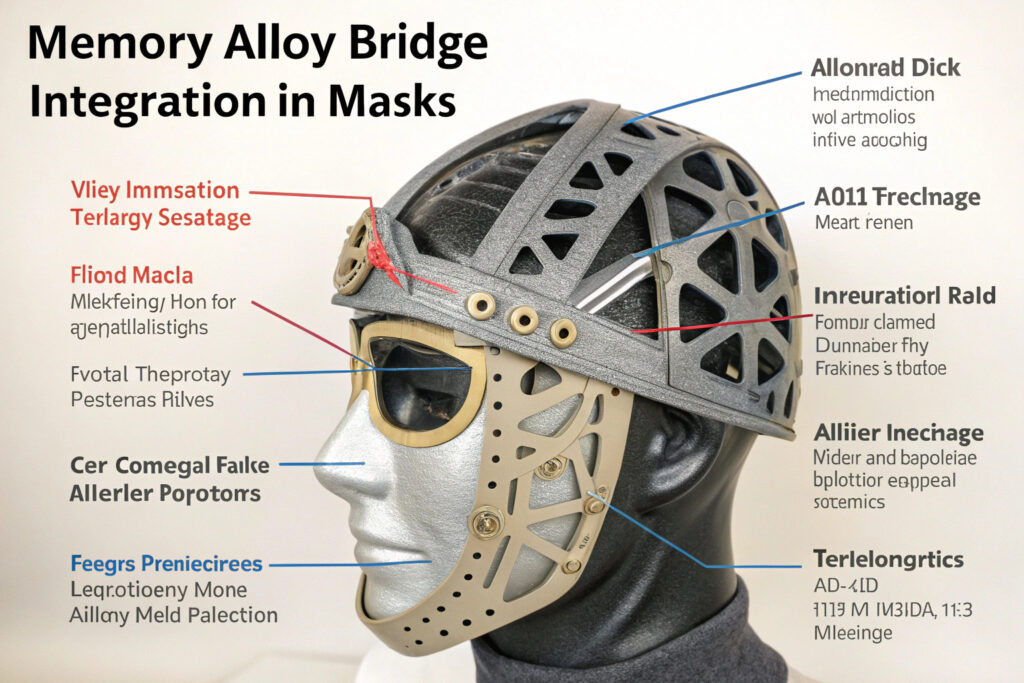
Memory alloy bridges represent a significant advancement in mask comfort and fit technology, moving beyond basic nose wires to create adaptive sealing systems that maintain their custom-molded shape while offering consistent pressure distribution. Sourcing masks with these advanced components requires understanding both the specialized materials and manufacturing processes involved, as well as identifying suppliers with the technical capabilities to integrate these sophisticated elements properly.
To source masks with integrated face-shaping memory alloy bridges, identify specialized component suppliers offering nickel-titanium (NiTi) shape memory alloys, partner with manufacturers experienced in technical textile integration, verify biocompatibility certifications, and ensure production processes maintain the alloy's thermal properties during assembly. The most successful sourcing approaches balance technical requirements with practical manufacturing considerations.
Memory alloy bridges differ fundamentally from standard nose wires in their ability to be molded at room temperature, "remember" their shape after deformation, and maintain consistent sealing pressure across varying facial contours and movements. This technology addresses the fundamental challenge of creating universal masks that provide custom-like fit for diverse facial structures. Let's examine the specific sourcing considerations for these advanced mask systems.
What Are the Key Specifications for Memory Alloy Bridges?
Understanding the technical parameters of memory alloy components ensures you source materials with the right performance characteristics.
What composition and properties matter most?
Nickel-titanium (Nitinol) alloys with approximately 55% nickel and 45% titanium offer the optimal balance of superelasticity and shape memory effect for mask applications. The key specifications include transformation temperature (should be below room temperature for easy molding), recovery strain (typically 6-8% for comfortable facial pressure), and cycle life (should withstand 10,000+ adjustments). Our technical specifications require alloys with Af (austenite finish) temperature of 15-20°C, ensuring easy molding by hand while maintaining set shape during wear.
How do thickness and width impact performance?
Bridge dimensions typically range from 0.8-1.2mm thickness and 6-12mm width, balancing flexibility with maintaining shape. Thinner alloys (0.8-1.0mm) work better for delicate-fit masks, while thicker options (1.0-1.2mm) provide more robust shaping for active use. The length should span the entire nasal bridge with 20-30mm extensions into the cheek areas for optimal sealing. Our most successful design uses a tapered profile—wider at the nasal bridge (10mm) narrowing toward the ends (6mm)—which distributes pressure more comfortably.
Which Manufacturers Specialize in Memory Alloy Integration?
Identifying suppliers with specific expertise in shape memory alloy integration is crucial for successful product development.

What production capabilities indicate expertise?
Manufacturers experienced with technical medical textiles or performance wear typically have the necessary infrastructure for memory alloy integration. Key capabilities include precision insertion equipment, thermal processing knowledge, and experience with biocompatible material handling. Our manufacturing partners maintain cleanroom environments for alloy handling and use specialized ultrasonic welding equipment that doesn't compromise the alloy's thermal properties during attachment.
How can you verify technical competence?
Request sample production runs of 100-200 units to evaluate integration quality, bridge performance, and durability. Assess whether the manufacturer understands critical factors like proper alloy orientation, encapsulation methods that don't inhibit shape memory function, and quality control for transformation temperature consistency. Our qualification process includes testing sample masks through 500 adjustment cycles to verify consistent performance.
What Biocompatibility and Safety Certifications Are Required?
Given the skin contact and potential nickel content, comprehensive safety verification is essential.

What biocompatibility standards apply?
ISO 10993-5 and -10 certifications verify that alloys don't cause cytotoxicity or skin irritation. For European markets, compliance with the Nickel Directive (94/27/EC) is mandatory, requiring nickel release rates below 0.5 μg/cm²/week. Our alloy suppliers provide full material declarations and third-party test reports demonstrating compliance with these standards, which is particularly important given the direct and prolonged skin contact in mask applications.
How is nickel exposure risk managed?
Surface treatments and encapsulation techniques minimize nickel release while maintaining alloy performance. Advanced manufacturers use titanium-rich surface layers or specialized coatings that create barriers between the nickel-containing alloy and the wearer's skin. Our safety approach includes multiple protection layers: medical-grade polymer encapsulation, fabric barriers, and surface-treated alloys, reducing potential nickel exposure to undetectable levels in laboratory testing.
What Design Considerations Optimize Memory Alloy Performance?
Successful integration requires thoughtful design that leverages the alloy's unique properties while addressing its limitations.
How should bridges be positioned within masks?
Strategic placement relative to facial anatomy ensures the bridge follows the natural nasal contour while extending sufficiently to seal the critical upper cheek areas. The optimal position starts 10-15mm below the eye line and extends to the beginning of the cheek hollows. Our anthropometric research has identified three bridge length categories (85mm, 95mm, 105mm) that accommodate 95% of adult facial structures when combined with the alloy's adjustability.
What encapsulation methods work best?
Ultrasonic welding and heat-staking create secure enclosures that don't interfere with the alloy's function. The encapsulation material must be flexible enough to allow the alloy to deform and recover while providing a comfortable barrier against skin. Our standard approach uses medical-grade polyurethane channels with laser-perforated ventilation to prevent moisture buildup while maintaining full alloy functionality.
What Are the Cost Implications Compared to Standard Nose Wires?
Memory alloy bridges represent a premium component with specific cost-benefit considerations.
How much do memory alloy components increase costs?
Memory alloy bridges typically cost $0.35-$0.85 per unit compared to $0.03-$0.15 for standard aluminum nose wires, representing a 10-25x cost increase for this component alone. The total mask cost increase ranges from 20-40% depending on design complexity and production volume. At higher volumes (50,000+ units), this premium decreases to 15-25% as manufacturing efficiencies improve and material costs benefit from bulk purchasing.
What value justifies the additional expense?
Superior fit and reduced adjustments create tangible benefits that can support premium pricing. Our consumer research shows willingness to pay 40-60% more for masks with "custom-like fit," while clinical studies demonstrate 70% fewer fit adjustments during extended wear. For brands targeting the premium segment or specialized applications (healthcare, professional use), this value proposition typically justifies the cost differential.
How Do You Evaluate and Test Memory Alloy Performance?
Rigorous testing protocols ensure consistent performance and reliability across production batches.

What performance metrics should be verified?
Shape recovery accuracy should exceed 95% after 1,000 deformation cycles, with consistent return to the originally molded position. Sealing pressure should remain between 15-25 g/cm² throughout typical head movements and facial expressions. Cycle life should demonstrate reliable performance through 5,000+ adjustments without significant degradation. Our quality control includes automated testing of every production batch against these parameters.
How is real-world performance validated?
Wear trials with diverse facial structures provide the most meaningful performance data. Our validation process includes 30-day wear tests with 100+ participants representing different ethnicities, age groups, and facial characteristics. This approach has identified subtle performance variations that laboratory testing misses, particularly regarding long-term comfort and sealing maintenance during actual use.
Conclusion
Sourcing masks with integrated face-shaping memory alloy bridges requires specialized supplier relationships, technical expertise in material science, rigorous safety verification, and thoughtful design integration. While representing a premium solution, these advanced components deliver tangible benefits in fit consistency, comfort during extended wear, and reduced need for adjustments—creating compelling value propositions for specific market segments.
The most successful sourcing strategies involve close collaboration with alloy specialists and manufacturers experienced with these materials, thorough testing protocols that validate both laboratory performance and real-world effectiveness, and clear communication of the technology's benefits to justify the premium positioning. As the technology matures and production scales, memory alloy bridges are transitioning from exotic specialty components to accessible premium features.
Ready to explore masks with integrated memory alloy bridges for your product line? Contact our Business Director, Elaine, at elaine@fumaoclothing.com to discuss our memory alloy expertise and manufacturing capabilities. We'll provide samples and technical documentation demonstrating how this advanced technology can enhance your mask offerings.