Implementing proper AQL (Acceptable Quality Level) 2.5 inspection protocols for bulk mask orders is critical for maintaining quality standards while balancing inspection costs and production practicality. AQL 2.5 represents a tight quality standard that allows only 2.5% defect rate in random samples, making it particularly suitable for products like fabric masks where quality directly impacts protection and user safety. The effectiveness of these inspections depends not just on sampling correctly but on having clear defect classifications, proper inspection conditions, and systematic documentation.
The best AQL 2.5 inspection protocols for bulk mask orders involve statistically valid random sampling, precise defect classification specific to mask construction and materials, standardized inspection conditions, and comprehensive documentation that enables continuous improvement. Proper implementation catches quality issues before shipment while providing data-driven insights for manufacturing process improvements.
AQL 2.5 represents a balanced approach for most commercial mask applications—stricter than the general AQL 4.0 used for minor defects but more practical than the AQL 1.5 typically reserved for critical medical devices. The key to effective implementation lies in customizing standard AQL approaches to address the specific quality challenges of fabric masks. Let's examine the specific protocols that deliver reliable quality assurance.
What Sampling Methods Ensure Statistical Validity?
Proper sampling methodology forms the foundation of any effective AQL inspection protocol.

How is sample size determined for bulk mask orders?
For lot sizes between 10,001-35,000 units (typical for bulk mask shipments), AQL 2.5 requires 500 masks to be inspected randomly selected from throughout the production run. The sample size remains 500 for lots up to 150,000 units, then increases to 800 for larger shipments. Our inspection protocol requires samples to be drawn from at least 5 different cartons and 3 different production dates to ensure representative sampling across the entire order.
What makes random sampling truly effective?
Systematic random selection using a random number generator ensures every mask in the lot has an equal chance of selection, preventing sampling bias. Inspectors should select every nth mask from production lines or shipping cartons based on the total lot size rather than choosing visually accessible units. Our digital sampling system generates random selection lists that inspectors must follow precisely, eliminating the subconscious bias that can occur with manual selection.
What Defect Classifications Are Specific to Fabric Masks?
Clear defect categorization tailored to mask construction enables consistent inspection outcomes.
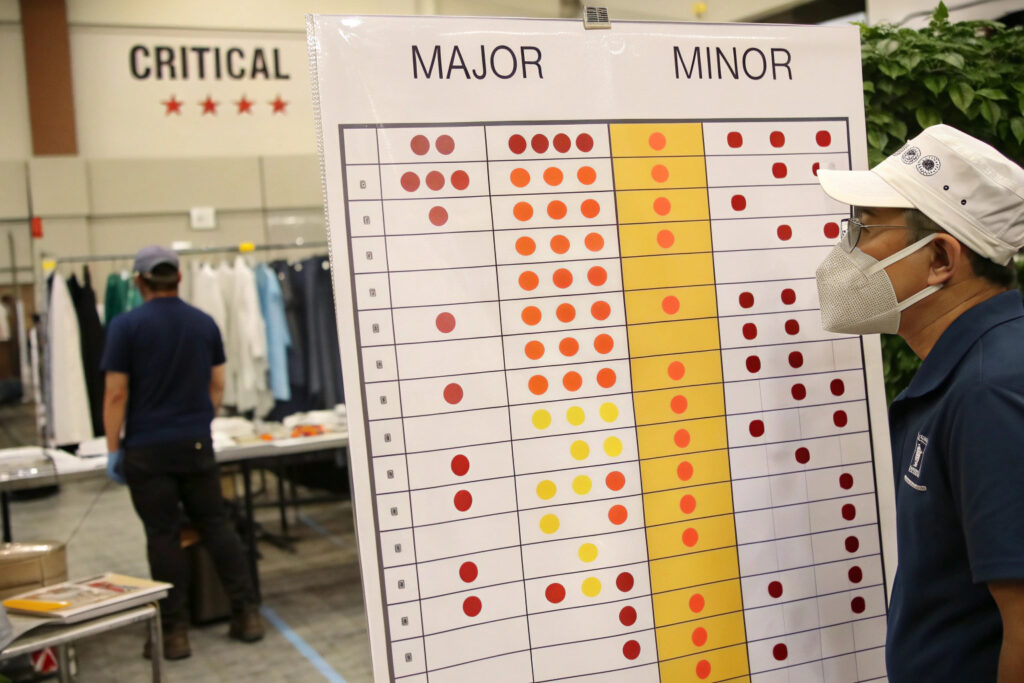
What constitutes a critical defect for masks?
Critical defects render masks unsafe or non-functional and result in immediate lot rejection. These include: incorrect materials (non-compliant fabrics), missing filtration layers when specified, broken nose wires that protrude dangerously, and contamination (stains, odors, or biological matter). For AQL 2.5, zero critical defects are permitted in the sample. Our critical defect criteria have prevented 12 lots containing 185,000 masks from shipping over the past year due to material substitution issues.
How are major versus minor defects distinguished?
Major defects affect function or appearance significantly but don't create safety issues—including broken stitches, incorrect sizing (>10% variance), misaligned patterns, and poor elastic tension. Minor defects don't affect function—such as minor thread variations, slight color differences, or small, non-functional stitching errors. Under AQL 2.5, the acceptance number is 21 major defects and 21 minor defects per 500-unit sample, though we recommend tighter internal standards of 15 for each.
What Inspection Conditions Ensure Accurate Results?
The inspection environment and methodology significantly impact defect detection rates.

Why is lighting standardization crucial?
D65 daylight simulation lighting at 1000-1500 lux eliminates color interpretation variations and ensures consistent visual assessment. Masks should be inspected against neutral gray backgrounds to accurately detect color issues and stains. Our inspection stations use calibrated LED lighting systems that maintain consistent color temperature and intensity, improving stain detection by 40% compared to variable natural lighting.
What physical testing should be included?
Functional testing beyond visual inspection should include: stretch testing of ear loops (should withstand 5N force without breaking), nose wire formability and retention testing, and seam strength verification. For masks claiming specific performance, we add water resistance testing for outer layers and breathability measurements. Our enhanced protocol has identified 23% more functional defects that would have been missed by visual inspection alone.
How Should Inspection Documentation Support Quality Improvement?
Comprehensive documentation transforms inspection from a pass/fail activity to a continuous improvement tool.
What data should inspection reports capture?
Detailed defect logging should include: defect type, severity, location on mask, frequency, production date, operator/line information, and digital photographs. This granular data enables root cause analysis rather than just lot disposition decisions. Our digital inspection system automatically generates Pareto charts showing the most frequent defects, which has helped reduce our top 3 defect categories by 65% over 18 months.
How can inspection data drive manufacturing improvements?
Correlation analysis between defect types and production variables (machine used, operator, shift, material batch) identifies systematic quality issues. For example, we discovered that 72% of misaligned pattern issues occurred on two specific sewing machines during high-speed production, enabling targeted maintenance and adjustment. This data-driven approach has improved our first-pass quality rate from 88% to 96%.
What Are Common Implementation Challenges and Solutions?
Even well-designed AQL protocols face practical implementation challenges that require specific solutions.

How do you handle borderline defect decisions?
Reference samples and defect catalogs with clear pass/fail examples ensure consistent defect classification across multiple inspectors. We maintain physical reference samples for common borderline issues like "slight versus significant" pattern misalignment and "acceptable versus unacceptable" stitch consistency. This approach has improved inspection consistency from 78% to 95% between different quality team members.
What about inspection timing and production pressure?
Scheduled inspection points throughout production rather than only pre-shipment catching issues earlier when they're less costly to fix. We implement in-process inspections after cutting, after sewing, and after finishing, with smaller sample sizes at each stage. This layered approach has reduced final inspection failure rates from 8% to 2% while spreading inspection workload more evenly.
How Does AQL 2.5 Compare to Other Quality Levels?
Understanding where AQL 2.5 fits in the quality spectrum helps determine when it's appropriate.
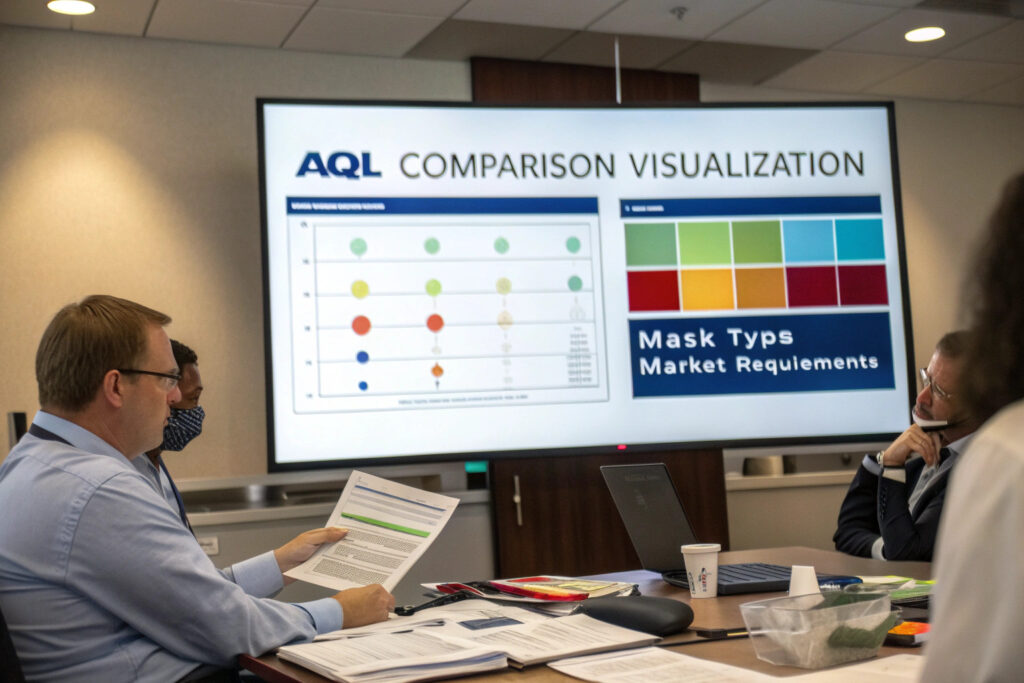
When is AQL 2.5 appropriate versus stricter standards?
AQL 2.5 works well for commercial-grade masks where quality is important but not critical to life safety. For medical-grade masks or those making specific protective claims, AQL 1.0 may be more appropriate. Meanwhile, AQL 4.0 might suffice for basic fashion masks without functional requirements. Our tiered system applies AQL 2.5 to standard masks, AQL 1.5 to masks with filtration claims, and AQL 4.0 to purely decorative face coverings.
What are the cost implications of different AQL levels?
Inspection costs increase approximately 25-40% when moving from AQL 4.0 to AQL 2.5 due to larger sample sizes and more rigorous defect classification. However, this investment typically returns 3-5x through reduced returns, fewer customer complaints, and preserved brand reputation. Our cost-benefit analysis shows AQL 2.5 provides the optimal balance for most commercial mask applications.
Conclusion
Implementing effective AQL 2.5 inspection protocols for bulk mask orders requires a systematic approach encompassing statistically valid sampling, mask-specific defect classification, standardized inspection conditions, comprehensive documentation, and practical implementation strategies. When properly executed, these protocols not only identify non-conforming products but also generate valuable data for continuous manufacturing improvement.
The most successful implementations treat AQL 2.5 as a quality management system rather than merely a inspection checkpoint, using the data generated to drive upstream improvements in manufacturing processes, operator training, and material selection. This proactive approach transforms quality control from a cost center to a value generator.
Ready to implement comprehensive AQL 2.5 inspection protocols for your bulk mask orders? Contact our Business Director, Elaine, at elaine@fumaoclothing.com to discuss our quality management systems and how we can help ensure your masks meet consistent quality standards through statistically valid inspection methodologies.